最新消息
為強化中小企業對智慧財產之瞭解,並提升專利、商標的申請品質及智財保護的能力,經濟部智慧局在與中華民國工業區廠商聯合總會合辦之113 年「營業秘密保護實
關於本局各項申請案件法定期間末日之計算,如遇特定紀念日放假,例如5月1日勞動節,依法務部函釋行政程序法第48條第4項的立法意旨為對於國定假日或休息日之
發文日期:中華民國113年4月18日發文字號:智商字第11350001820號主旨:公告新增包括「商標註冊申請案加速審查申請書」、「商標代理人登錄申請
為讓優秀的專利得獎作品能持續在國內外市場發光發熱,經濟部智慧財產局今年4月於「台北國際汽機車零配件展」(4/17-4/20南港一館)及「台灣國際創意禮
新電子申請系統於113年4月18日起提供更新程式下載,本次擴增功能包括「行動自然人憑證登入」、「圖式製作小工具新增版本更新通知」及「匯出臨櫃送件檔」功
國際動態
根據《2023年歐洲專利指數》,2023年公司和發明人向歐洲專利局(EPO)提交了19萬9,275件專利申請,申請件數創新高,比前一年增長了2.9%(圖1),延續了2022年的成長率2.6%和2021年的4.7%。 圖 1 EPO受理發明專利申請件數&nbs
美國專利商標局(USPTO)和美國著作權局(下合稱為主管當局)發布了關於非同質化代幣(NFTs)的智慧財產(IP)法律和政策影響的聯合研究結果。 在聯合研究期間,兩局透過調查通知徵求公眾意見,舉行了三次公開圓桌會議,並檢視了現有的文獻和判例法。在報告中,主管當局認可評論者的
德國專利商標局(DPMA)於2024年3月18日發布人工智慧(AI)創新活動的評估和分析。數據顯示,AI在生活許多領域中發揮重要作用,相關創新活動持續增加。 2023年,以AI為重點的技術領域中,德國的有效專利公開申請數量比五年前增加40%(如圖1)。AI的核心領域成長趨勢
研討會資訊
多媒體
- 產業協力專利審查面詢試行作業方案簡介

- 【創新研發教學推廣影片系列】商標申請及運用的策略性思考

- 【創新研發教學推廣影片系列】企業容易誤觸之著作權侵權態樣

- 【創新研發教學推廣影片系列】營業秘密之侵害類型與救濟方式

- 【創新研發教學推廣影片系列】商標的基本概念介紹

- 【創新研發教學推廣影片系列】營業秘密管理機制的建立

- 【創新研發教學推廣影片系列】從實際用途盤點常見智權

- 【企業專利應用影片系列】 - 「phase 4:專利加值應用創造額外價值」

- 【企業專利應用影片系列】 - 「phase 3:統合專專利布局優化與精實專利管理」

- 【企業專利應用影片系列】 -「 phase 2:統合專利能量建立權利保護機制」


 專利
專利 商標
商標 著作權
著作權






























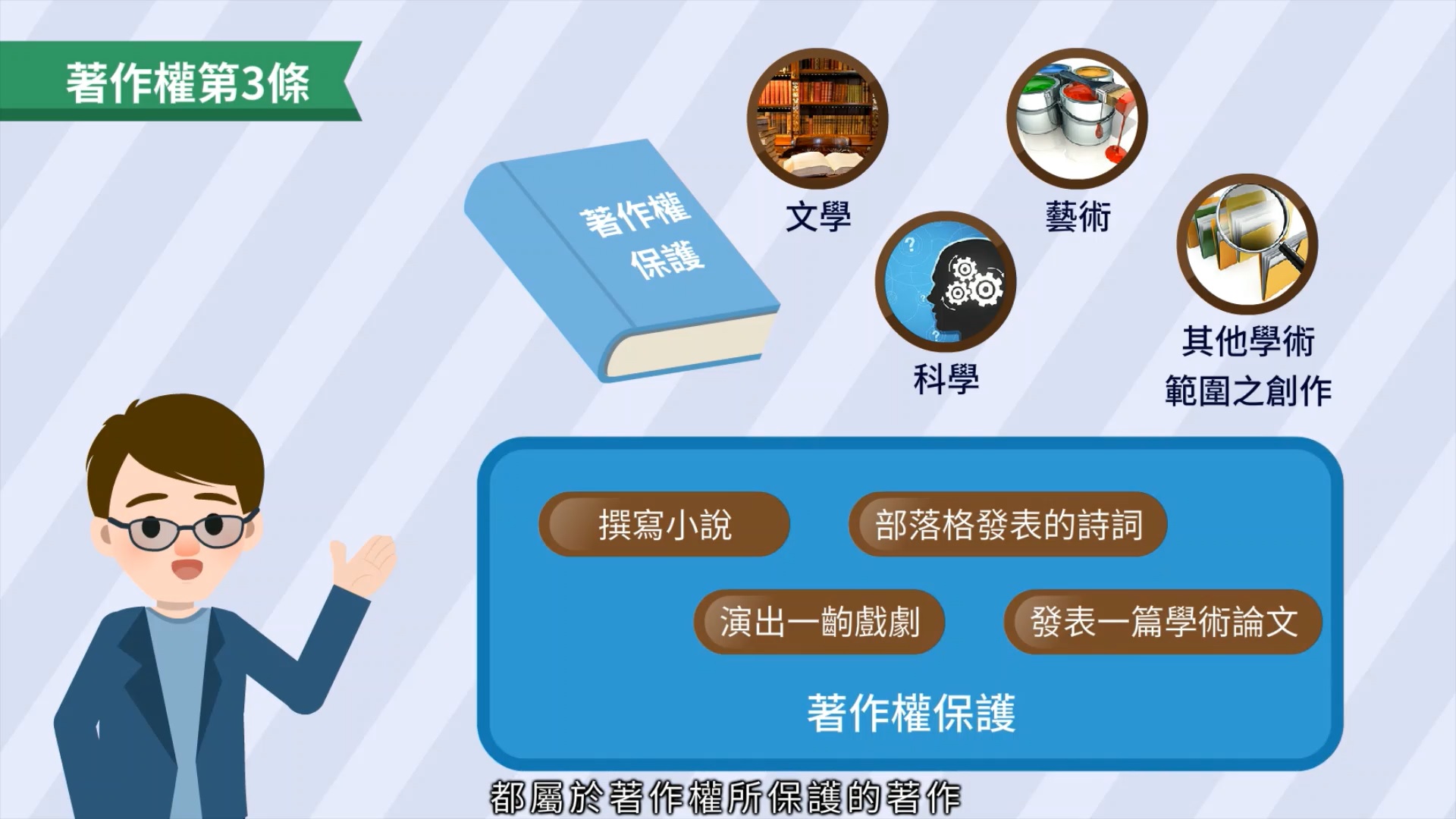



 網站導覽
網站導覽 常見問答
常見問答 意見信箱
意見信箱 雙語詞彙
雙語詞彙